The Purpose and Aim
Most current climate models assume that secondary organic aerosol (SOA) in the atmosphere is purely light scattering and non-absorbing. However, recent advances suggest that aging transforms components of non-absorbing SOA into optically active compounds, often collectively known as “brown carbon” (BrC), which absorb solar radiation1 (see Fig. 1) and affect climate forcing. The chemical processes underlying SOA aging and the subsequent formation and transformation of BrC in the atmosphere are not well understood. It is postulated that reactive nitrogen, acidity and water-mediated chemistry may play key roles in the browning of SOA and hence contribute to the global radiative forcing budget. These possibilities, and others, will be explored in detail in the proposed studies.
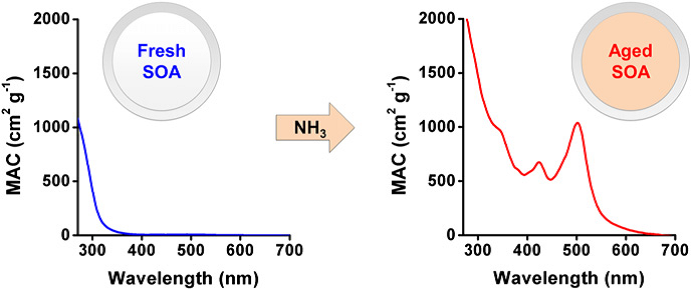
Figure 1: Mass absorption coefficients (MAC) of fresh and aged limonene-derived SOA in the UV/visible light spectrum, showing formation of light-absorbing BrC2.
The overall aim of this project is to elucidate molecularlevel processes and properties involved in formation and transformation of BrC in the atmosphere. Specifically to:
- Unravel key chemical pathways responsible for BrC formation during SOA aging mediated by reactive nitrogen (NOx, NH3, HNO3), aerosol acidity (H2SO4) and water chemistry;
- Identify and quantify the chromophores or classes of similar compounds – key browning agents – in fresh and aging SOA;
- Characterize roles of recently identified highly oxidized multifunctional molecules (HOMs) in SOA browning;
- Develop process-level models of effects of reactive nitrogen, aerosol acidity and water on the molecular composition and properties (thermodynamic and optical) of evolving BrC-SOA mixtures in the atmosphere;
- Formulate parameterizations to improve radiative forcing predictions in regional and global climate models.
This will substantially improve abilities to meet increasing demands for robust climate change predictions, and facilitate development of effective climate mitigation strategies, thereby alleviating major economic, environmental and societal problems (see below).
Background:
Atmospheric aerosols markedly affect the radiative balance in Earth’s atmosphere and play key roles in climatic processes3. Major fractions of atmospheric aerosols consist of carbonaceous aerosols — organic carbon (OC) and black carbon (BC, commonly known as “soot”)4,5. Soot potentially warms the planet as it strongly absorbs light, while OC has traditionally been largely treated in most climate models as a light-scattering, and thus cooling, substance. The cooling due to this scattering partly offsets the warming by co-emitted BC from combustion processes6. The molecular composition of OC and its evolution during various processes of atmospheric aging have been extensively studied in the past decade4,5,7-11. However, despite various advances, our understanding of the climate-related properties of atmospheric OC and its effects on both the atmospheric environment and climate forcing are far from complete. An important fundamental aspect is the need to understand the behavior of the OC fraction in various complex mixing states that strongly absorbs light, popularly called Brown Carbon12 (BrC). OC can be emitted directly through combustion processes (primary organic aerosol – POA) or formed in the atmosphere by gas-to-particle conversion processes (SOA) such as nucleation, condensation and heterogeneous and multiphase chemical reactions evolves continuously into extremely low volatility, highly oxidized multifunctional molecules (HOMs) and macro-molecules with high molecular weight5,13-15, which may be light absorbing2,12,16-18.
reactions of isoprene on acidic aerosol particles [Limbeck et al., 2003]; aqueous photochemistry of pyruvic acid in
the presence of atmospheric electrolytes (e.g., SO
4
2
and NH
4
+
)[Rincon et al., 2009, 2010]; and reaction of
isoprene epoxydiols onto sulfate aerosol to form absorbing oligomers [Lin et al., 2014]. Few field measurements
have been able to link these mechanisms and products directly to brown carbon aerosol [Zhang et al., 2011;
Mohr et al., 2013; Lin et al., 2014]
Both primary and secondary sources of BrC aerosols have been identified, including combustion of fossil fuels19, biomass burning20,21, biological aerosols (e.g. soil humics and bioaerosols)22, and SOA formed from anthropogenic or biogenic precursors12. While the primary BrC sources are straightforward to identify, mechanisms that generate light-absorbing SOA are highly uncertain10. Candidates include: nitration of polycyclic aromatic hydrocarbons, yielding light-absorbing nitrophenols23,24; reactions of NH3, NH4+, or amino acids with condensed-phase carbonyl products2,12,16,25-27; acid-catalyzed aldol condensation of volatile aldehydes28,29; reactions of OH radicals with aromatic hydroxyacids and phenols in cloud water30,31; heterogeneous reactions of isoprene on acidic aerosol particles32; aqueous photochemistry of pyruvic acid in the presence of atmospheric electrolytes (e.g., SO42- and NH4+)33,34; and reactions of isoprene epoxydiols on sulfate aerosol forming absorbing oligomers35. The complexity of resultant light-absorbing organic compounds and variations in their relative concentrations complicate attempts to characterize the molecular composition of BrC and identify the molecules or aggregates governing its optical properties. Despite these analytical difficulties, several classes of compounds have been identified as potential contributors to BrC’s light absorption, including nitroaromatic compounds, such as nitrophenols, imidazole-based and other N-heterocyclic compounds, and quinones. Less is known about the molecular composition of light-absorbing oligomers produced through condensation reactions and humic like substances (HULIS)12. It has also been proposed that supramolecular aggregates and complexes of organic molecules with transition metals may be responsible for BrC’s observed optical properties. However, it is unclear if typical BrC includes a few strong chromophores or diverse weak chromophores. The latter would be more consistent with the featureless UV−visible spectra of BrC observed in most field studies. However, analyses of BrC’s spectra in some field studies have identified clear peaks in the visible range36-38, indicating that both molecular and supramolecular chromophores may coexist in ambient BrC. Several laboratory proxies of BrC, have also been shown to exhibit distinct absorption spectra (such as the 510 nm band in the aged limonene SOA shown in Figure 1). The identification and structural characterization of BrC chromophores clearly require highly sensitive molecular approaches capable of detecting both strongly and weakly absorbing species (as illustrated in Figure 2). Quantifying BrC’s contribution to light absorption is critically important for accurate interpretation of aerosols’ optical depth (AOD), the atmospheric column’s light extinction due to both scattering and absorption.
Understanding the environmental effects of BrC, its sources, formation, and atmospheric aging mechanisms requires robust description of relationships between its molecular composition and light absorption properties. Available evidence suggests that even a very small weight fraction of strongly absorbing BrC chromophores may substantially affect OA’s optical properties10. Because of the low concentrations of light-absorbing molecules in the complex organic mixtures present in both laboratory-generated and ambient OA, identifying BrC chromophores is highly challenging10. A recent review12 clearly indicates that various BrC chromophores may be present in both POA and SOA originating from diverse sources. However, knowledge of their chemical identities, light-absorbing properties, concentrations, and atmospheric stability is fragmentary. Thus, we have insufficient understanding for practical parametrization of BrC properties in atmospheric and climate models. To obtain sufficient observations and scientific knowledge for constraining and validating future models there it is a need to do investigations that include fundamental molecular level studies of BrC chromophores, including characterization of their chemical composition and optical properties, sources, formation mechanisms, and atmospheric degradation
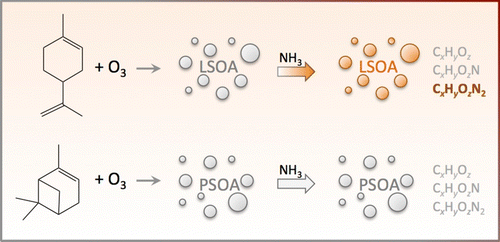
Figure 2: Molecular analysis of secondary organic aerosol (SOA) generated from ozonolysis of two structural monoterpene isomers: D-limonene SOA (LSOA) and α-pinene SOA (PSOA). Laboratory simulated aging of LSOA and PSOA, through conversion of carbonyls into imines mediated by NH3 vapors in humid air, resulted in browning of the LSOA sample, while the PSOA sample remained white.16
Project Description:
As outlined in the above literature review, formation of secondary BrC in the atmosphere is poorly understood. However, we postulate that it is promoted by local abundance of reactive nitrogen (e.g. NOx, NH3, HNO3), acidity (H2SO4) and water through processing of VOCs in the atmosphere. Further, we suggest there are three main potential sources of secondary BrC: processing of emissions from fossil-fuel (FF) combustion, biomass burning (BB) and biogenic ecosystems (BG). As illustrated in Fig. 3, typical FF combustion emissions, e.g. from coal-fired power plants, are rich in VOCs, NOx and SO2, while burning of agricultural fields (BB) generates high levels of VOCs, NOx and NH3. Emissions from vegetation (BG) are thought to be rich in VOCs and water vapor. Atmospheric oxidation and aging leads to the formation of substantial amounts of BrC from these sources that may significantly contribute to global radiative forcing.
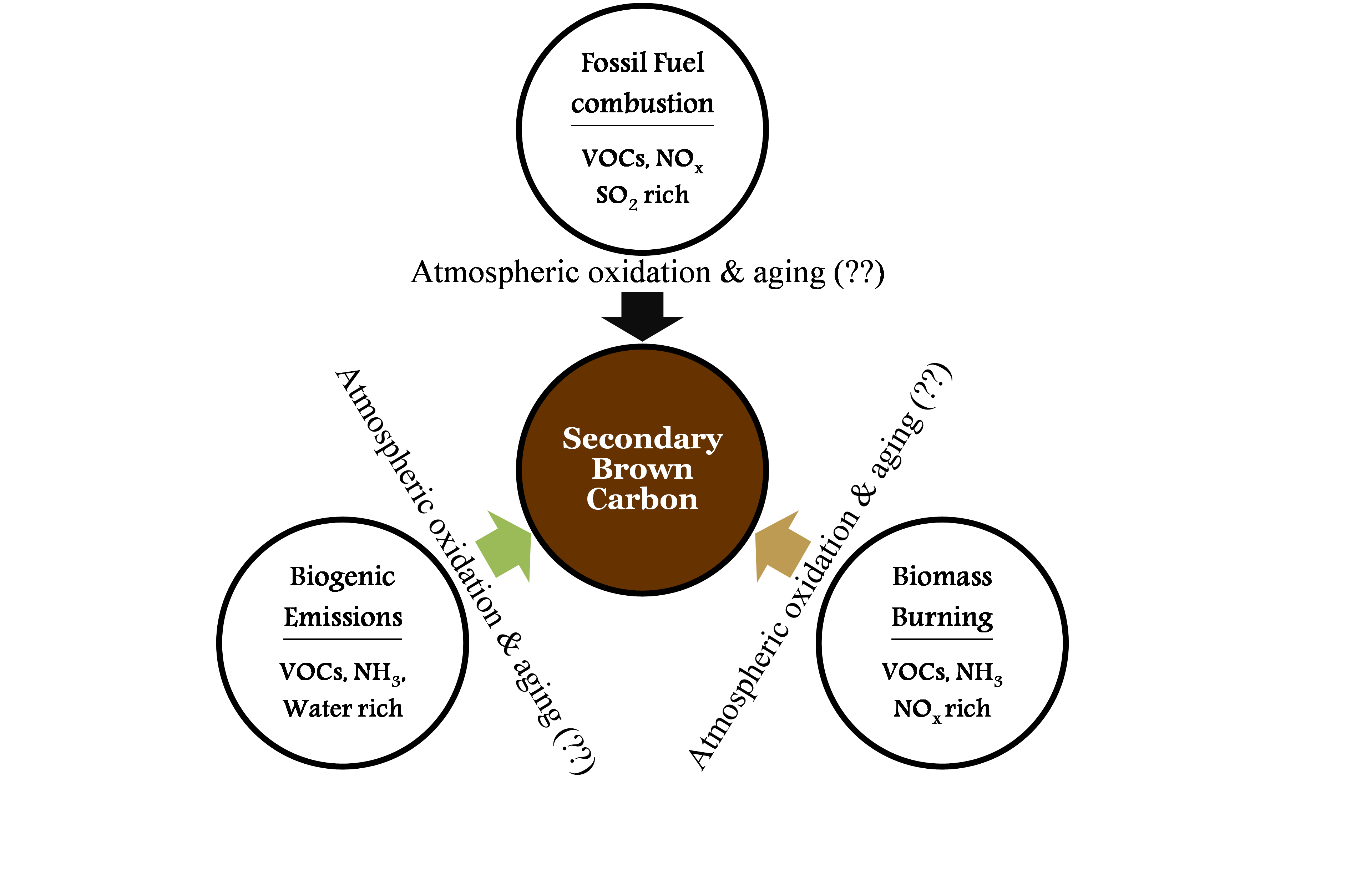
Figure 3: Non-light-absorbing SOA from various sources is transformed into BrC during atmospheric aging: we will explore the important, unknown (??) atmospheric oxidation and aging processes involved
3.1 Methods: The first steps will be to confirm indications that reactive nitrogen, acidity and water-influenced chemical processes are involved in transformation of colorless SOA into light-absorbing BrC. Thus, we will examine in detail effects of RH, NH3, NOx and acidic sulfate (H2SO4) on fresh and aged SOA derived from oxidation of atmospherically relevant biogenic and anthropogenic VOCs (e.g. limonene, isoprene, benzene, toluene and ethene) using University of Gothenburg’s internationally unique aerosol experimental facility (G-FROST: Gothenburg Flow-tube Reactor for Oxidation Studies at low Temperature) equipped with state-of-the-art instruments (see the Equipment section).
We will identify and quantify components of SOA as it ages, especially the chromophores: the molecules/atoms responsible for its potential browning. In the G-FROST facility, we will simulate formation of secondary BrC from the three suggested major atmospheric sources: FF, BB and BG (see Fig. 3). The experimental matrix for SOA formation and aging that will be applied in each case is described in the Research tasks section, and a flow diagram of the experimental set-up is shown in Fig. 4.
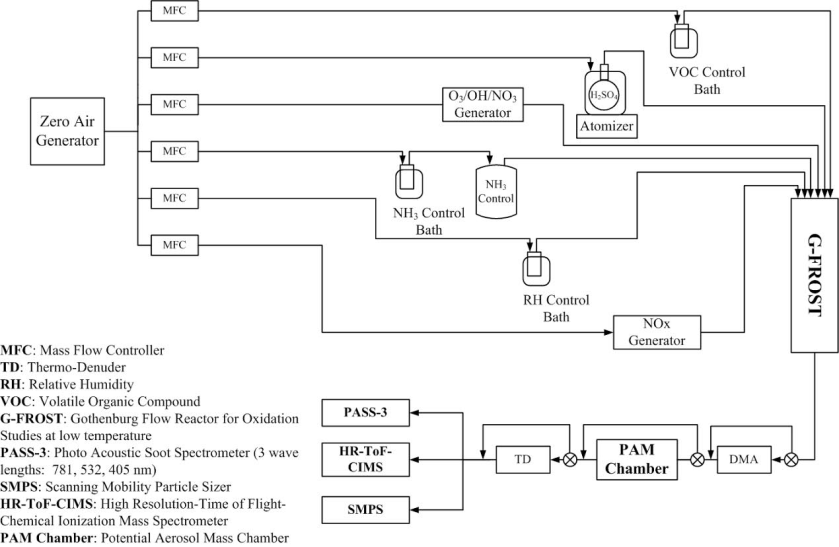
Figure 4: Schematic diagram of simulations of SOA aging and BrC transformations at the G-FROST facility (University of Gothenburg).
The first experimental step will be generation of SOA from typical VOCs (e.g. benzene and toluene from FF and BB; limonene, a-pinene and isoprene from BG) using O3, OH and NO3 as key oxidants in G-FROST. This will be followed by selection of aerosols with targeted particle sizes (e.g. 100 nm) using a differential mobility analyzer (DMA), intense artificial aging in a potential aerosol mass (PAM) chamber representing a few days aging in the atmosphere39, and finally characterization of the generated SOA. The PAM chamber is designed to maximize SOA oxidation, but we aim to control its oxidizing capabilities to mimic various stages of SOA oxidation. We will also characterize fresh SOA. The molecular composition of SOA and other oxygenated compounds in gas phase will be determined and resolved using our High‐Resolution Time-of-Flight‐Chemical Ionization Mass Spectrometer (HR‐ToF‐CIMS). We will aim to identify chromophores, the browning agents, in both the fresh and aged SOA (including the gas phase) generated in all experiments. In parallel efforts, the size distributions, volatility, and optical properties (absorption and scattering) of fresh and aged SOA will be measured using a scanning mobility particle sizer (SMPS), volatility tandem differential mobility analyzer (VTDMA) and a 3-wavelength photo acoustic soot spectrometer (PASS-3), respectively. A major experimental challenge in this project will be to control the influential variables during the SOA generation and aging processes tightly. A schematic diagram of the integrated procedure using our experimental set-up is presented in Fig. 4. We plan to conduct experiments at standard temperature (25oC) and pressure (1 atmosphere) conditions (STP). The RH in the flow systems will be regulated according to the aim of each experiment.
3.2 Research Tasks: The following tasks are planned (with timing indicated in Table 1), in order to characterize effects of various co-emitted pollutants on the formation, aging, composition (e.g. chromophore formation) and optical properties of SOA derived under the designated sets of conditions. These measurements at G-FROST equipped with standard characterization instruments (SMPS, CPC, gas analyzers, humidity, pressure and temperature controls) and state-of-the-art instrumentation (VTDMA, PASS-3, HR -ToF-CIMS) will provide mechanistic understanding of observed changes in composition and optical properties of the SOA, enabling parameterization (Task 3) and comparison with data acquired from chamber studies (Task 2).
Task 1A: Fossil Fuel combustion conditions (Toluene-, Ethene-, NOx-, SO2-rich):
Under typical FF combustion conditions, the experimental matrix will include:
VOCs — Toluene and Ethene; Oxidants — O3, OH, NO3.
(i) Characterization of fresh SOA (bypassing the PAM and Thermodenuder, TD, in Fig. 4) treated at:
- High NOx (with/without (w/wo) H2SO4 seeds) and low/high RH;
- High NOx with H2SO4 (w/wo NH3) and low/high RH
(ii) Aging of SOA in the PAM chamber (bypassing the TD; Fig.4) and characterization following treatments outlined in (i).
(iii) Characterization of fresh and aged SOA (w/wo bypassing the PAM in Fig. 4) at volatility temperatures selected using the TD – with DMA, TD and SMPS in series, following treatments outlined in (i) & (ii). This will enable us to correlate optical properties of BrC and molecular components at the selected volatility temperatures.
Task 1B: Biomass Burning conditions (Ethene-, Benzene-, NOx-, NH3-rich):
Under typical BB conditions, the experimental matrix will include:
VOCs — Benzene and Ethene; Oxidants — O3, OH, NO3
Fresh and aged SOA treated at (i) high NOx (w/wo NH3) and low/high RH, (ii) high NOx (w/wo H2SO4 seeds) and low/high RH, and a selection of volatility temperatures using the TD as described in Task1A for mapping SOA/BrC composition and optical properties.
Task 1C: Biogenic Emissions (Monoterpenes- and NH3-rich):
Under typical biogenic ecosystem (BG) conditions, the experimental matrix will include:
VOCs — Limonene, Isoprene; Oxidants — O3, and OH
Fresh and aged SOA treated at high NOx (w/wo NH3) and low/high RH will be characterized selected volatility temperatures using the TD as in Task 1A for mapping SOA/BrC composition and optical properties.
Task 2: Smog chamber experiments will be used for aging SOA (up to 48 hr) and assessing its effects on the resulting SOA-BrC mixtures’ thermodynamic (volatility) and optical (absorption and scattering) properties. Selected experiments described in Task 1(A, B &C) will be repeated and extended, with similar instruments to those in the G-FROST facility using the 10 m3 temperature-controlled smog chamber at Carnegie Mellon University (CMU), USA. Findings from these experiments will be evaluated against results from the G-FROST SOA aging experiments described in Task 1. Similarities and discrepancies will be analyzed and used for designing new experiments and parameterizations for climate models.
Task 3: Theoretical work – information from G-FROST and smog chamber experiments on the composition and properties of fresh and aged aerosols resulting from SOA-BrC mixtures will be used to formulate parameterizations for climate models. BrC parameterizations will be developed using the new “volatility basis set” vapor pressure-fitting scheme40, covering a wide range of vapor pressures (10-2 to 105 µgm-3), and applied to the SOA transformation processes and BrC optical properties. To acquire reliable, high quality laboratory data for field and model evaluations, we will follow steps outlined in the extensive review by Co-applicant Hallquist and colleagues5. Findings from Tasks 1&2 will also be used to constrain models derived to explain data obtained from our Chinese and Indian field studies (see significance section) in collaboration with China project modeling work package leader Simpson at Chalmers Technical University, Sweden. Specific emphasis will be on development of models applied in MERGE (ModElling the Regional and Global Earth system): a Swedish strategic research area, focusing on biosphere-atmosphere interactions. The main applicant (Pathak) and co-applicant Hallquist are coordinating the University of Gothenburg’s contribution to MERGE and Hallquist is heading the research initiative Terrestrial carbon cycle and aerosol–cloud–climate interaction, with collaborators including MERGE partners from Lund University and Chalmers (The EMEP model expert Simpson). There will be further major synergies with CLEO (CLimate change and Environmental Objectives), a Swedish EPA research program hosted by the Swedish Environmental Research Institute – IVL (2010-2016), in which Hallquist is leading efforts to deliver SOA parameterizations for models used by SMHI (e.g. the MATCH model in combination with RCA) and Chalmers (the EMEP model) related to climate change and Swedish Environmental Objectives.
Thus, the project will be highly relevant to reactive nitrogen- and acidity-rich environments in the world, e.g. major hotspots of biomass burning in NH3-rich rural agricultural settings, and both NOx- and SO2– (acidity) rich urban centers. Important examples include areas of developing countries such as China and India, where our ongoing research related to biomass burning and emissions from fossil fuel combustion — e.g. NOx, NH3, O3, smog, SOA and Black Carbon (BC) — will synergistically facilitate the proposed research, e.g. the 2.7 M SEK U-Forsk project BC and O3 in rural Indian sites on the Indo Gangetic Plain funded by VR (2.7 M SEK) and headed by Main Applicant Pathak, and Photochemical smog in China, a VR-funded framework project (25 M SEK) headed by Co-applicant Hallquist. Massive air pollution occurs in these major hotspots, with significant fractions of BrC (e.g. brownish haze)41 from both primary and secondary sources.
Significance:
4.1 Scientific contributions:
This project will improve molecular level understanding of SOA aging and interlinked BrC formation processes and its properties. Effects of chromophores and HOMs on the light-absorbing potential of evolving SOA-BrC mixtures in the atmosphere will be updated in current climate models. The project will also contribute to the national strategic research project MERGE and the Swedish EPA project CLEO, thereby strengthening transfer of knowledge from the laboratory to practical (policy-making) sectors. Knowledge and insights from this project will significantly enhance our long-term scientific investigations in India and China, where needs for information regarding the focal issues are greatest. Thus, the project will facilitate advances in the treatment of SOA aging and BrC as short-lived climate forcers within Swedish and international abatement strategies, e.g. photochemical smog of China and climatic perturbations by Asian brown clouds (ABC) in the Indian subcontinent, largely derived from burning biomass. It will also strengthen Swedish research activities on SOA headed by Hallquist, soot-SOA interactions led by Pathak and ice nucleation research headed by co-applicant Pettersson with an extensive high-profile international network.
4.2 Societal value of the research:
This project will have extremely high societal value, by providing molecular level knowledge of the composition and processes of formation and transformation of atmospheric brown carbon. More than 90% of people in rural areas of low- and middle-income nations burn biomass to meet their daily energy needs, thereby exacerbating severe associated problems. Women and children in kitchens are most vulnerable, being exposed to extremely high levels of smoke several hours a day. However, much greater knowledge of the chemical agents formed and transformed in SOA is required, in order to direct research on their health and ecological effects (links to genetic mutations and respiratory diseases etc.), and identify ways to counter the problems. Brownish smogs also pose threats (notably to asthma patients) in many urban areas (e.g. Beijing smoggy haze). Mechanistic understanding of the key agents in SOAs (rural and urban) is clearly essential to develop effective mitigation strategies. Thus, findings from this research on SOA-BrC mixtures’ composition, evolution and properties will open avenues for greatly improving air quality globally and thus enhancing sustainable development, human well-being and ecological health.
References:
(1) Andreae, M. O. and Gelencsér, A. (2006) Atmos. Chem. Phys., 6, 10, 3131-3148.
(2) Updyke, K. M. et al. (2012) Atmospheric Environment, 63, 0, 22-31.
(3) IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
(4) Kanakidou, M. et al. (2005) Atmospheric Chemistry and Physics, 5, 1053-1123.
(5) Hallquist, M. et al. (2009) Atmospheric Chemistry and Physics, 9, 14, 5155-5236.
(6) Myhre, G. et al. (2013) Atmos. Chem. Phys., 13, 4, 1853-1877.
(7) Ervens, B. et al. (2011) Atmos. Chem. Phys., 11, 21, 11069-11102.
(8) Herckes, P. et al. (2002) Environmental Science & Technology, 36, 22, 4777-4782.
(9) Carlton, A. G. et al. (2009) Atmos. Chem. Phys., 9, 14, 4987-5005.
(10) Kroll, J. H. and Seinfeld, J. H. (2008) Atmospheric Environment, 42, 16, 3593-3624.
(11) Rudich, Y. et al. (2007) Annual Review of Physical Chemistry, 58, 1, 321-352.
(12) Laskin, A. et al. (2015) Chemical Reviews.
(13) Kalberer, M. (2004) Science, 303, 5664, 1659-1662.
(14) Ehn, M. et al. (2014) Nature, 506, 7489, 476-479.
(15) Ehn, M. et al. (2012) Atmos. Chem. Phys., 12, 11, 5113-5127.
(16) Laskin, J. et al. (2014) Environmental Science & Technology, 48, 20, 12047-12055.
(17) Saleh, R. et al. (2013) Atmos. Chem. Phys., 13, 15, 7683-7693.
(18) Zhong, M. and Jang, M. (2014) Atmos. Chem. Phys., 14, 3, 1517-1525.
(19) Bond, T. C. (2001) Geophysical Research Letters, 28, 21, 4075-4078.
(20) Kirchstetter, T. W. et al. (2004) Journal of Geophysical Research: Atmospheres, 109, D21, n/a-n/a.
(21) Lack, D. A. et al. (2012) Proceedings of the National Academy of Sciences, 109, 37, 14802-14807.
(22) Andreae, M. O. and Crutzen, P. J. (1997) Science, 276, 5315, 1052-1058.
(23) Pitts, J. et al. (1978) Science, 202, 4367, 515-519.
(24) Jacobson, M. Z. (1999) Journal of Geophysical Research: Atmospheres, 104, D3, 3527-3542.
(25) Bones, D. L. et al. (2010) Journal of Geophysical Research: Atmospheres, 115, D5, n/a-n/a.
(26) Sareen, N. et al. (2010) Atmos. Chem. Phys., 10, 3, 997-1016.
(27) Flores, J. M. et al. (2014) Atmos. Chem. Phys., 14, 11, 5793-5806.
(28) Nozière, B. and Esteve, W. (2005) Geophysical Research Letters, 32, 3, L03812.
(29) Nozière, B. and Esteve, W. (2007) Atmospheric Environment, 41, 6, 1150-1163.
(30) Gelencsér, A. et al. (2003) Journal of Atmospheric Chemistry, 45, 1, 25-33.
(31) Hoffer, A. et al. (2004) Geophysical Research Letters, 31, 6, L06115.
(32) Limbeck, A. et al. (2003) Geophysical Research Letters, 30, 19, 1996.
(33) Rincón, A. G. et al. (2010) The Journal of Physical Chemistry Letters, 1, 1, 368-373.
(34) Rincón, A. G. et al. (2009) The Journal of Physical Chemistry A, 113, 39, 10512-10520.
(35) Lin, G. et al. (2014) Journal of Geophysical Research: Atmospheres, 119, 12, 7453-7476.
(36) Liu, J. et al. (2013) Atmos. Chem. Phys., 13, 24, 12389-12404.
(37) Corr, C. A. et al. (2012) Atmos. Chem. Phys., 12, 21, 10505-10518.
(38) Doherty, S. J. et al. (2010) Atmos. Chem. Phys., 10, 23, 11647-11680.
(39) Kang, E. et al. (2007) Atmos. Chem. Phys.
(40) Donahue, N. M. et al. (2006) Environmental Science & Technology, 40, 8, 2635-2643.
(41) Ramanathan, V. et al.: Atmospheric Brown Clouds: Regional Assessment Report with Focus on Asia. United Nations Environment Programme: Nairobi, Kenya, 2008.
(42) Jonsson, Å. M. et al. (2008) Atmos. Chem. Phys.
(43) Jonsson, Å. M. et al. (2006) Environmental Science & Technology, 40, 1, 188-194.
(44) Pathak, R. K. et al. (2012) Environ Sci Technol, 46, 21, 11660-9.
(45) Lopez-Hilfiker, F. D. et al. (2013) Atmos. Meas. Tech. Discuss., 6, 5, 9347-9395.
